What is corrosion?
1. Concept of Corrosion (Rust)
Corrosion is the chemical and electrochemical reaction between metal and its environment, which results in the formation of corrosion products on the metal surface. The most common form of corrosion is rusting, which involves the chemical or electrochemical reaction on the metal surface, causing the metal to enter the oxidized (ionic) state. This reaction significantly reduces the mechanical properties such as strength and toughness of the metal, destroys the geometric shape of the metal components, increases wear between parts, and shortens the lifespan of the metal.
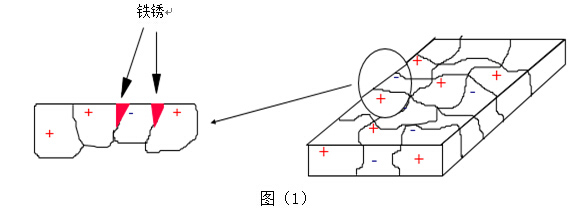
2. Types of Corrosion
Corrosion can be classified into the following types based on its form and environment:
Uniform corrosion: Corrosion occurs uniformly on the metal surface.
Galvanic corrosion / Bimetallic corrosion: Corrosion that occurs when different metals come into contact.
Pitting corrosion: Localized corrosion that forms small holes or pits.
Creep corrosion: Corrosion that occurs at the gaps or joints of metals.
Corrosion of steel in concrete: Corrosion of steel in concrete structures.
Crevice corrosion: Localized corrosion caused by the flow of liquids.
Stress corrosion: Corrosion that occurs under stress.
3. Steel Corrosion
Steel corrosion is primarily caused by the electrochemical inhomogeneity of the steel surface, generating a potential difference, which leads to the formation of different electrodes (micro-cells) and causes the anode part to corrode. This corrosion can be explained and controlled by electrochemical principles.
4. Corrosion Environments
Corrosion can occur in various environments, including but not limited to:
Corrosion in the atmosphere: Corrosion caused by moisture and pollutants in the air.
Corrosion in industrial environments: Such as flue gas, waste gas, wastewater, acid and alkali corrosion, high-temperature oxidation, etc.
Corrosion in soil and underground: Corrosion of underground pipelines and structures.
Corrosion in marine environments: Corrosion in seawater.
Corrosion under special conditions: Such as corrosion under high temperature, high pressure, and other special conditions.
5. Coating Protection
Anti-corrosion coatings protect metals from corrosion through the following mechanisms:
Barrier protection: A barrier layer is formed on the substrate surface to prevent seawater or other corrosive substances from contacting the substrate.
Cathodic protection: The coating itself serves as a sacrificial anode, providing electrochemical protection.
Inhibition, passivation: The coating passivates the steel surface and forms a passivation layer on it.
6. Conclusion
Corrosion is a complex process involving various factors and environmental conditions. Understanding the types of corrosion, the environments, and protective measures is crucial for extending the lifespan of metal structures. By using appropriate anti-corrosion measures, such as anti-corrosion coatings, corrosion can be effectively reduced, protecting the integrity and functionality of metal structures.